TiO₂ Information
What is TiO₂
TiO₂ is a very stable material physically and chemically, and has superior coverage and staining power because it has the highest refractive index among the white pigments, as well as accurate particle sizes and dispersibility. It is used to remove gloss from chemical textiles, to improve the abrasion resistance of chemical textiles, and for pigmentation. It is also widely used in various other areas, such as electronic materials, a glass, and welding rod coverings, based on its electronic characteristics.
As new products utilizing the characteristics of TiO₂ are currently rapidly being developed and applied to various areas, the competition among countries is becoming increasingly fierce. The representative products are catalysts and photocatalysts using nano-type products.
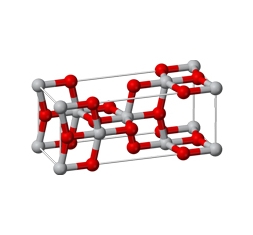

History of TiO₂
As researches on the manufacture of Ti (titanium metal) and TiO₂ (titanium dioxide) have been conducted since the 19th century, W. J. Kroll established an engineering method for titanum metal. Anatase TiO₂ was commercialized by Fabriques de Produits Chimques de Thann of France in 1923, through multiple researches, and has been continuously developed technically.
At present, the TiO₂ that is being commercially distributed is largely divided into two categories: rutile and anatase. Rutile was first manufactured by NL Industries (U.S.) at the end of 1940.
Perspectives of TiO₂
A wide array of products using TiO₂ as well as white pigment are being developed and applied, which will increase the manufacture and consumption of high-value-added derivatives. The representative product is the photocatalyst TiO₂, which is present around us. The photocatalyst TiO₂ is now being applied to daily life due to the rapid technical development and expansion of its applications. Its relevant market size will also significantly grow.
Its annual global output and consumption is about 5 million tons per year, and prominent chemical groups, such as DuPont, Crystal, and Tronox, are manufacturing it. In South Korea, Cosmo Chemical is the sole manufacturer of TiO₂ and thus competes with global firms.
Type and Applications
Sulfate Process
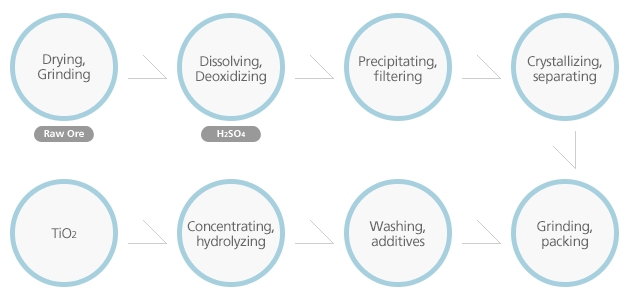
Drying,Grinding
Raw ore
The ore is first dried, ground, and classified, and is then dissolved in a sulfate solution FeTiO₃ + 2H₂SO₄ > FeSO₄ + TiOSO₄ + 2H₂O

Dissolving,Deoxidizing
After the deoxidation of Fe³+ and Fe²+ in a sulfate solution consisting of Ti and Fe elements, the solution is then filtered and cooled to crystallize the coarse Ferrous Sulfate Solution heptahydrate (FeSo4 7H₂O), which is separated from the process. The solution is hydrolyzed to produce insoluble white hydrous titanium oxide.TiOSO₄ + 2H₂O에서TiO(OH)₂ + H₂SO₄

The first particle size and distribution
This is an important process for deciding the first particle size and distribution of hydrous titanium oxide, which is needed to come up with good products with appropriate particle sizes in the hydrolysis process. The pulp is extensively washed to remove the residual traces of metallic impurities. The washed pulp is then calcinated in a rotary kiln, and ground. Then the final products are manufactured. TiO(OH)₂> TiO₂ + H₂O

Chloride process
In the sulfate process, rutile can be produced as using catalyst added before calcinations or on additives in hydrolysis step. Thus, this process can produce either anatase or rutile crystal forms depending on additives used prior to calcinations differently from the chloride process.
Note: The grades of rutile and anatase are diversified by purpose, by coating with aluminum (AI) or silica (Si) in the post-treatment process.
